Safety Profile
Trajenta®
(Linagliptin)
Unique CVOT Program.3-6


According to the Diabetes Atlas, there are more than 530 million adult diabetes patients around the world12. Undoubtedly, there is significant diversity across this population. Given such diverse patient situations, understanding the safety profile of a treatment is important. That is why the long-term CV and kidney safety profile of Trajenta® has been studied in more than 13,000 T2D patients.3-6 In fact, Trajenta® is backed by one of the most unique and robust CVOT programs, which included patients with relatively early T2D as well as more severe patients at high risk for CV and/or kidney disease.
Furthermore, specific prespecified subgroup analyses have continued to highlight the CV and kidney safety profile of Trajenta® in a range of patient populations, including younger and older patients, Asian patients, and patients with normal and reduced kidney function.
Patients with Established CV and/or Kidney Disease.3-4
CARMELINA® included nearly 7,000 T2D patients with established CV and/or kidney disease, and HbA1c levels from 6.5% up to 10%. In CARMELINA®, Trajenta® demonstrated a long-term CV and kidney safety profile in the overall population.3-4 Compared to placebo, Trajenta® did not increase the risk of adverse CV events (3P-MACE), adverse kidney events, or risk of HHF, even in patients at high risk of heart failure. These outcomes remained largely consistent across various subgroups.

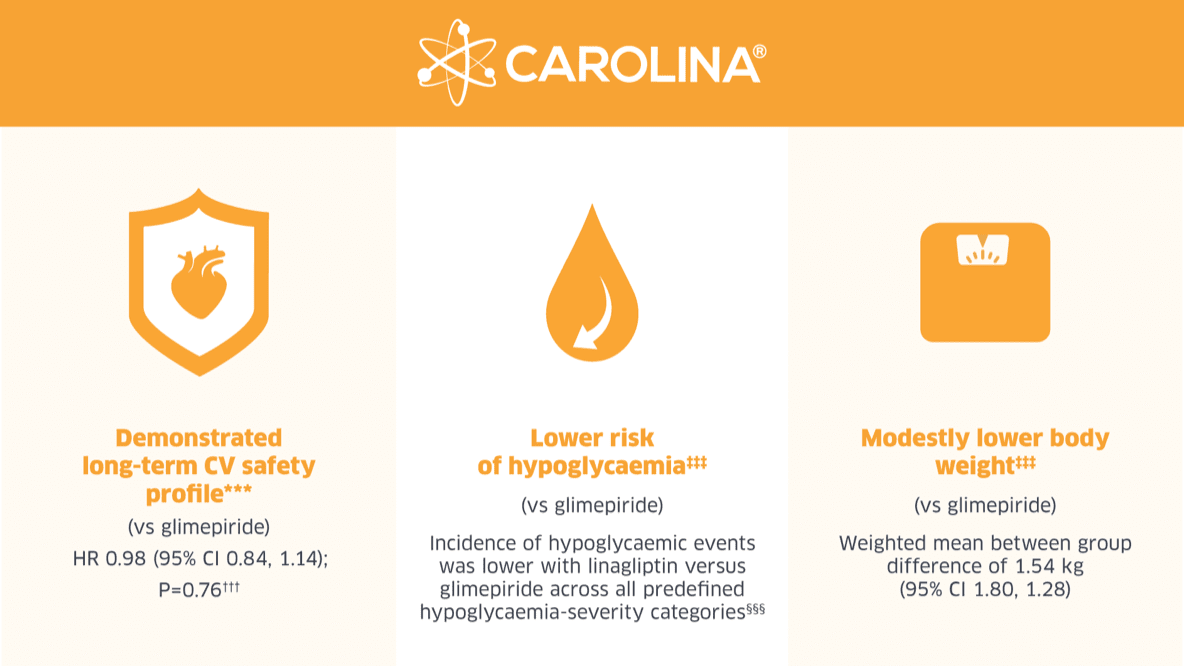
Relatively Early T2D Patients.5-6
CAROLINA® is the only CVOT to compare a DPP4i to an active comparator. It included about 6,000 patients with relatively early T2D and increased CV risk. In CAROLINA®, Trajenta® demonstrated its long-term CV safety profile as well as a lower risk of hypoglycaemic events and weight gain in the overall population5-6 and in prespecified subgroups2 compared to a sulphonylurea (glimepiride).
Note: Trajenta® is not indicated for the prevention of hypoglycaemic events nor for the prevention of weight gain.
In a Nutshell…5-6
Trajenta® is a DPP4i with data unique data set. Its CVOT program included two dedicated CVOTs: CAROLINA® - specifically designed to assess the CV safety of Trajenta® in early T2D patients at increased CV risk (mean HbA1c of 7.2% and mean eGFR above 75.0)§, and CARMELINA® - specifically designed to assess the CV and kidney safety of Trajenta® in T2D patients with established CV and/or kidney disease (mean HbA1c of 8.0% and mean eGFR below 55.0).‡ In addition, Trajenta® is the only DPP4i whose CVOT program proactively assessed kidney safety as a secondary endpoint in the pre-specified testing hierarchy. When you need a DPP4i, choose one that has a robust set of safety data. Choose the Simplicity of Trajenta®.
DPP4i |
Number of CVOT Trials |
CVOT |
Assessed CV Safetya |
Risk of HHF Compared to Placebo HR (95% CI) |
Assessed Kidney Safetyb |
Baseline HbA1c
|
Mean eGFR
|
|---|---|---|---|---|---|---|---|
|
Vildagliptin |
None |
- |
- |
- |
- |
- |
- |
|
Saxagliptin7 |
1 |
SAVOR-TIMI |
|
1.27 (1.07, 1.51) |
- |
6.5% to 12.0% (8.0%) |
Saxagliptin: 72.5 |
|
Alogliptin8,9 |
1 |
EXAMINE |
|
1.19 (0.9, 1.58) |
- |
6.5% to 11.0% (8.0%) |
NRh |
|
Sitagliptin10 |
1 |
TECOS |
|
1.00 (0.83, 1.19) |
- |
6.5% to 8.0% (7.2%) |
Overall: 74.9 |
|
Linagliptin3,6 |
2 |
CARMELINA3 |
|
0.90 (0.74, 1.08) |
|
6.5% to 10.0% (8.0%) |
Linagliptin: 54.7 |
|
CAROLINA6 |
|
- |
- |
6.5% to 8.5% (7.2%) |
Linagliptin: 76.5 |
Extensive Subgroup Analysis of CVOT Patients

Review the CARMELINA® subgroup analysis by age

Review the Asian subgroup analysis
Adverse Events
Trajenta® has an established safety and tolerability profile.11 The risk of hypoglycaemia with linagliptin alone is low and the incidence was comparable to placebo. The adverse drug reactions for linagliptin as described in the EU SmPC are provided in the table below.
Adverse reactions by treatment regimen
System organ class (Adverse reaction) | Trajenta® |
|---|---|
Infections and infestations (Nasopharyngitis) | Uncommon |
Immune system disorders (Hypersensitivity) | Uncommon |
Respiratory, thoracic and mediastinal disorders (Cough) | Uncommon |
Gastrointestinal disorders (Pancreatitis) | Raree |
Gastrointestinal disorders (Constipation) g | Uncommon |
Skin and subcutaneous tissue disorders (Angioedema) c | Rare |
Skin and subcutaneous tissue disorders (Urticaria) c | Rare |
Skin and subcutaneous tissue disorders (Rash) c | Uncommon |
Skin and subcutaneous tissue disorders (Bullous pemphigoid) | Raree |
Metabolism and nutrition disorders (Hypoglycaemia) f | Very common |
Investigations (Amylase increased) | Uncommon |
Investigations (Lipase increased) d | Common |
Very common: ≥ 1/10, common: ≥ 1/100 to <1/10, uncommon: ≥ 1/1,000 to < 1/100, rare: ≥ 1/10,000 to < 1/1,000, very rare: <1/10,000 or not known: cannot be estimated from the available data.
Footnotes
-
CI: confidence interval; CV: cardiovascular; CVOT: cardiovascular outcome trial; DPP4i: dipeptidyl peptidase-4 inhibitor; eGFR: estimated glomerular filtration rate; ESRD: end stage renal disease; HHF: hospitalization for heart failure; HR: Hazard ratio; MI: myocardial infarction; T2D: type 2 diabetes
-
*
The CARMELINA® primary endpoint is time to first occurrence of any of the following components: CV death, non-fatal MI, non-fatal stroke. The key secondary endpoint is time to first occurrence of any of the following components: death due to kidney disease, sustained ESRD or a sustained decrease of ≥40% in eGFR from baseline.
-
†
The CAROLINA® primary endpoint is time to first occurrence of any of the following components: CV death, non-fatal MI and non-fatal stroke. The key secondary efficacy endpoint is proportion of patients on-treatment and maintaining HbA1c ≤7.0% at final visit without the need for rescue medication, without any moderate or severe hypoglycaemic episodes and without >2% weight gain between end of titration and final visit.
-
‡
CARMELINA® included patients with high CV and renal risk. High CV risk was defined as a history of coronary artery disease, stroke or peripheral vascular disease, and microalbuminuria or macroalbuminuria, defined as urinary albumin:creatinine ratio (UACR) higher than 30 mg/g or equivalent; high renal risk was defined as (1) estimated glomerular filtration rate (eGFR) of 45 to 75 mL/min/1.73 m2 and UACR higher than 200 mg/g or equivalent or (2) eGFR of 15 to 45 mL/min/1.73 m2 regardless of UACR. Participants with end-stage renal disease, defined as an eGFR less than 15 mL/min/1.73 m2 or requiring maintenance dialysis, were excluded. The mean baseline HbA1c for patients receiving linagliptin was 7.9% and for patients receiving placebo was 8.0%. The mean baseline eGFR for patients receiving linagliptin was 54.7 and for patients receiving placebo was 54.5.
-
§
CAROLINA® included patients with early type 2 diabetes and high CV risk. High cardiovascular risk was defined as (1) established atherosclerotic cardiovascular disease (documented ischemic heart disease, cerebrovascular disease, or peripheral artery disease), (2) multiple risk factors (at least 2 of the following: type 2 diabetes duration > 10 years, systolic blood pressure > 140 mm Hg [or receiving at least 1 blood pressure–lowering treatment], current smoker, low-density lipoprotein cholesterol ≥ 135 mg/dL [3.5 mmol/L], or receiving lipid-lowering treatment), (3) age at least 70 years, and (4) evidence of microvascular complications (impaired kidney function [estimated glomerular filtration rate of 30-59 mL/min/1.73m2], urine albumin/creatinine ratio ≥ 30 μg/mg, or proliferative retinopathy).
-
#
The primary endpoint was time to first occurrence of any of the following components: CV death, non-fatal MI, non-fatal stroke. The primary endpoint occurred in 434/3,494 (12.4%) and 420/3,485 (12.1%) patients in the linagliptin and placebo groups, respectively (HR: 1.02 (95% CI, 0.89, 1.17); p < 0.001 for non-inferiority; p = 0.74 for superiority).
-
**
HR for time to 3P-MACE based on Cox regression analyses in patients treated with at least 1 dose of study drug. Median observation time was 2.2 (IQR, 1.5-2.9) years for Trajenta® and 2.2 (IQR, 1.5-2.8) years for placebo. The primary aim was to establish noninferiority of linagliptin compared with placebo for time to 3PMACE, defined by the upper limit of the 2-sided 95% CI for the HR of linagliptin relative to placebo being less than 1.3. A sequentially rejective multiple test procedure was applied, first testing the primary hypothesis of noninferiority for linagliptin (p<0.001), and, only if this first test was significant, followed by 2 parallel confirmatory superiority tests (not statistically significant; p = 0.74 for the 3P-MACE and p = 0.62 for the composite kidney outcome).
-
††
Hospitalisation for heart failure occurred in 209/3,494 (6.0%) vs 226/3,485 (6.5%) patients in the linagliptin and placebo groups, respectively (HR: 0.90 (95% CI, 0.74, 1.08) p=0.26). Because both of the parallel confirmatory superiority tests (primary: p=0.74 for 3P-MACE; secondary: p=0.62 for composite kidney outcome) were null, findings for this outcome should be interpreted as exploratory.
-
‡‡
HR based on Cox regression analyses in patients treated with at least 1 dose of study drug.
-
§§
The key secondary endpoint was time to first occurrence of any of the following components: Death due to kidney disease, sustained ESRD or a sustained decrease of ≥40% in eGFR from baseline. The key secondary kidney endpoint occurred in 327/3,494 (9.4%) and 306/3,485 (8.8%) patients in the linagliptin and placebo groups, respectively (HR: 1.04 (95% CI 0.89, 1.22) p=0.62).
-
##
HR for time to secondary kidney endpoint based on Cox regression analyses in patients treated with at least 1 dose of study drug. Median observation time was 1.9 (IQR, 1.2-2.6) years for linagliptin and 1.7 (IQR, 1.2-2.5) years for placebo.
-
***
The CAROLINA® primary endpoint was defined as non-inferiority of Trajenta® vs glimepiride in time to first occurrence of CV death, non-fatal MI, or non-fatal stroke. The primary endpoint occurred in 356/3,023 (11.8%) and 362/3,010 (12.0%) patients in the linagliptin and glimepiride groups, respectively (HR: 0.98 (95% CI, 0.84, 1.14); p < 0.001 for non-inferiority; p = 0.76 for superiority).
-
†††
P value for superiority. HR for time to 3P-MACE based on Cox regression analyses in patients treated with at least 1 dose of study drug.
-
‡‡‡
Because the test for superiority of the primary endpoint was null, findings for the secondary outcomes should be interpreted as exploratory.
-
§§§
Percentage of patients experiencing a hypoglycaemic event was 10.6% for linagliptin and 37.7% for glimepiride (HR 0.23 (95% CI, 0.21, 0.26) non-inferiority p<0.0001). Kaplan-Meier estimate; hazard ratio and 95% CI derived from Cox regression with factor treatment; 2-sided p-value.
-
a
CVOT proactively assessed long-term cardiovascular outcomes as a primary endpoint in the pre-specified testing hierarchy, in-line with guidance for assessing cardiovascular safety of drugs for the treatment of type 2 diabetes, issued by the U.S. Food and Drug Administration (FDA) in December, 2008.
-
b
Refers to kidney safety investigated in a long-term randomised clinical trial with pre-specified, statistically powered, confirmatory tested for superiority, and adjudicated hard kidney outcomes (e.g., composite of death due to kidney disease, progression to sustained ESKD, or sustained eGFR decrease by ≥40%) in a population enriched for CKD (by specific CKD enrolment criteria).
-
c
Based on post-marketing experience
-
d
Based on lipase elevations >3xULN observed in clinical trials
-
e
Based on the linagliptin cardiovascular and renal safety study (CARMELINA®)
-
f
Adverse reaction observed in combination with metformin plus sulphonylurea.
-
g
Adverse reaction observed in combination with insulin.
-
h
Mean eGFR not reported; median eGFR at baseline for patients randomized to alogliptin was 71.1 ml/min/1.73 m2 and for patients randomized to placebo was 71.2 ml/min/1.73 m2.
References
-
Cooper M, et al. Diabetes Obes Metab. 2020; 1–12.
-
Kadowaki, T, et al. Diabetol Int. 2020. doi.org/10.1007/s13340-020-00447-5.
-
Rosenstock J, et al. JAMA. 2019;321:69-79.
-
Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39.
-
Marx N, et al. Diab Vasc Res. 2015;12:164-74.
-
Rosenstock J, et al. JAMA. 2019; doi:10.1001/jama.2019.13772.
-
Scirica BM et al. N Engl J Med 2013;369:1317
-
White WB et al. N Engl J Med 2013;369:1327
-
Zannad F et al. Lancet 2015;385:2067
-
Green JB et al. N Engl J Med 2015;373:232
-
Trajenta® Singapore Prescribing Information.
-
International Diabetes Federation, IDF Diabetes Atlas, diabetesatlas.org; Accessed July 5, 2022.






